Workshop "Standard-compliant Software Development"
 The mobile apps for patient-centered research used in the study conducted in UNITI will be developed according to the principles of the Medizinproduktegesetz MPG and the new EU Medical Device Regulation MDR. At the invitation of the Chair of Clinical Epidemiology and Biometry of the Medical Faculty of the University of Würzburg, Winny Schlee and Axel Schiller from Regensburg took part in the training course "Normgerechte Softwareentwicklung im international regulierten Umfeld (DE, EU, USA)" (Standard-compliant software development in an internationally regulated environment (DE, EU; USA)), in which Marc Holfelder (LA2 GmbH), member of the external UNITI Advisory Board, who presented the most important regulatory aspects of the MPG with regard to software development to the UNITI partners from Regensburg and Würzburg.
The mobile apps for patient-centered research used in the study conducted in UNITI will be developed according to the principles of the Medizinproduktegesetz MPG and the new EU Medical Device Regulation MDR. At the invitation of the Chair of Clinical Epidemiology and Biometry of the Medical Faculty of the University of Würzburg, Winny Schlee and Axel Schiller from Regensburg took part in the training course "Normgerechte Softwareentwicklung im international regulierten Umfeld (DE, EU, USA)" (Standard-compliant software development in an internationally regulated environment (DE, EU; USA)), in which Marc Holfelder (LA2 GmbH), member of the external UNITI Advisory Board, who presented the most important regulatory aspects of the MPG with regard to software development to the UNITI partners from Regensburg and Würzburg.

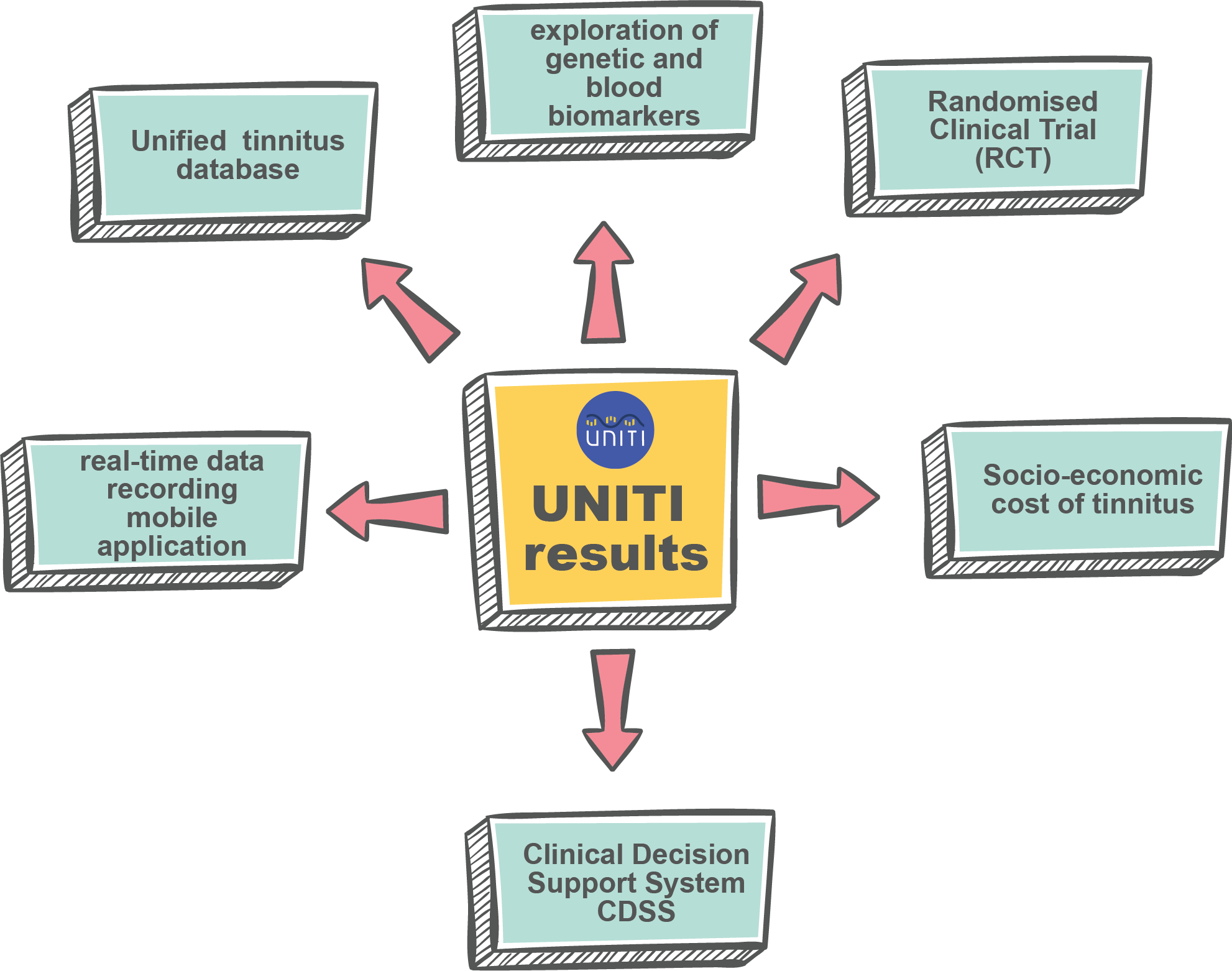

 One of the main objectives of UNITI is to conduct a large scale multicentre Randomised Clinical Trial (RCT) with a combination of interventions. Five clinical centres (partners of UNITI consortium) will have a target of recruiting 100 patients each over a period of 9 months.
One of the main objectives of UNITI is to conduct a large scale multicentre Randomised Clinical Trial (RCT) with a combination of interventions. Five clinical centres (partners of UNITI consortium) will have a target of recruiting 100 patients each over a period of 9 months. 

