The mobile apps for patient-centered research used in the study conducted in UNITI will be developed according to the principles of the Medizinproduktegesetz MPG and the new EU Medical Device Regulation MDR. At the invitation of the Chair of Clinical Epidemiology and Biometry of the Medical Faculty of the University of Würzburg, Winny Schlee and Axel Schiller from Regensburg took part in the training course "Normgerechte Softwareentwicklung im international regulierten Umfeld (DE, EU, USA)" (Standard-compliant software development in an internationally regulated environment (DE, EU; USA)), in which Marc Holfelder (LA2 GmbH), member of the external UNITI Advisory Board, who presented the most important regulatory aspects of the MPG with regard to software development to the UNITI partners from Regensburg and Würzburg.
The mobile apps for patient-centered research used in the study conducted in UNITI will be developed according to the principles of the Medizinproduktegesetz MPG and the new EU Medical Device Regulation MDR. At the invitation of the Chair of Clinical Epidemiology and Biometry of the Medical Faculty of the University of Würzburg, Winny Schlee and Axel Schiller from Regensburg took part in the training course "Normgerechte Softwareentwicklung im international regulierten Umfeld (DE, EU, USA)" (Standard-compliant software development in an internationally regulated environment (DE, EU; USA)), in which Marc Holfelder (LA2 GmbH), member of the external UNITI Advisory Board, who presented the most important regulatory aspects of the MPG with regard to software development to the UNITI partners from Regensburg and Würzburg.
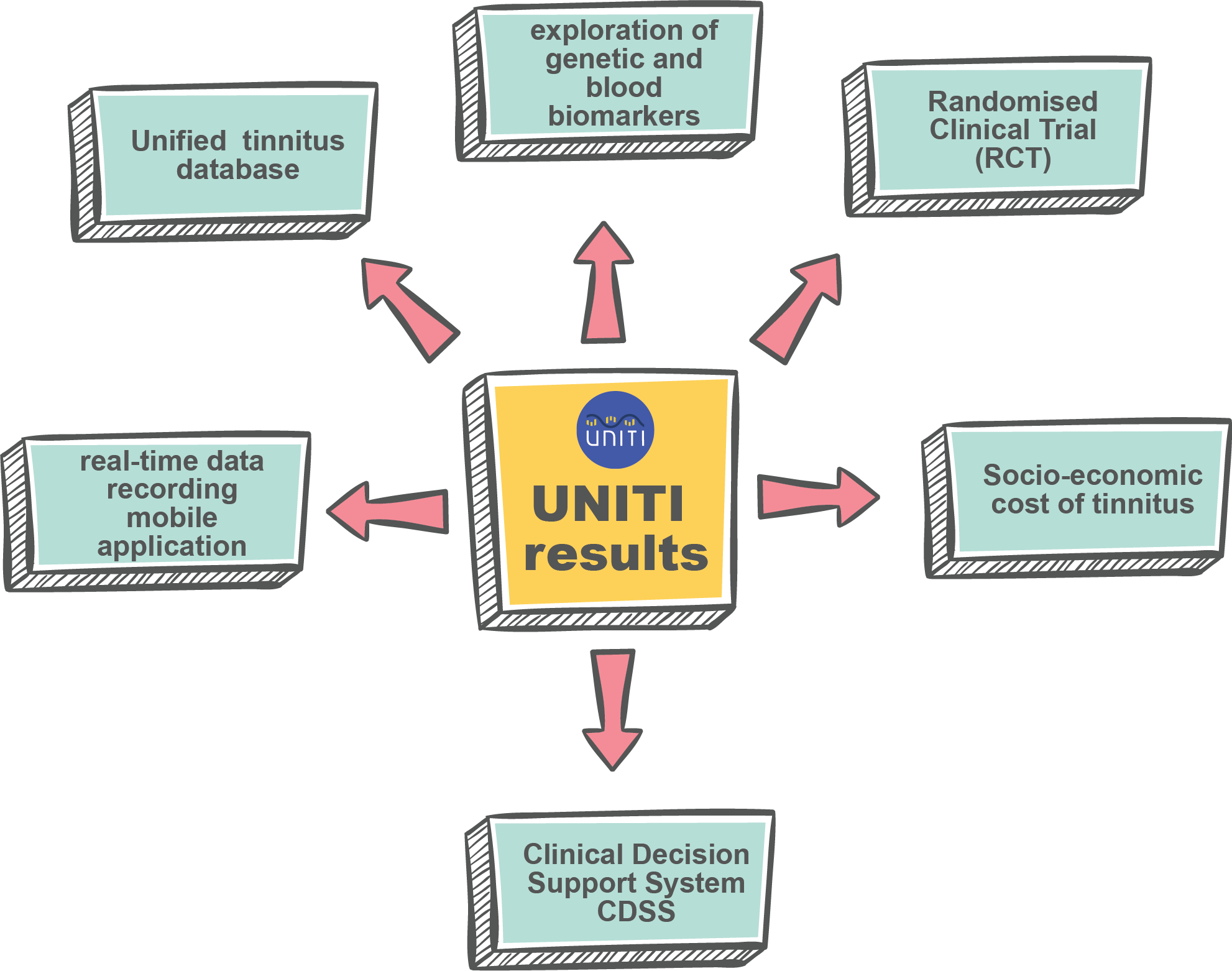
UNITI results
UNITI project finished in September 2023. It’s overall aim was to deliver a predictive computational model based on existing and longitudinal data attempting to address the question which treatment approach is optimal for a specific patient based on specific parameters. Clinical, epidemiological, medical, genetic and audiological data, including signals reflecting ear-brain communication, were analysed from existing databases. Predictive factors for different patient groups were extracted and their prognostic relevance were tested in a randomized controlled trial (RCT) in which different groups of patients underwent a combination of therapies targeting the auditory and central nervous systems.
Click here to learn more about the UNITI results.