Brain, hearing and Tinnitus science
 A special Topic on Brain, hearting and Tinnitus science has now been published on the MDPI website: https://www.mdpi.com/topics/Brain_Hearing_and_Tinnitus_Science
A special Topic on Brain, hearting and Tinnitus science has now been published on the MDPI website: https://www.mdpi.com/topics/Brain_Hearing_and_Tinnitus_Science
With this multidisciplinary Topic, UNITI consortium wants to generate an open access forum for clinical and translational tinnitus research, including preclinical studies, innovative diagnostic procedures, and novel treatment strategies that aim to improve the patient journey from diagnosis to the best-suited therapeutic approach for the individual patient. In this respect, research papers on individualized treatment decisions and decision support systems are also strongly encouraged. Original research papers and systematic reviews of high quality are welcome.

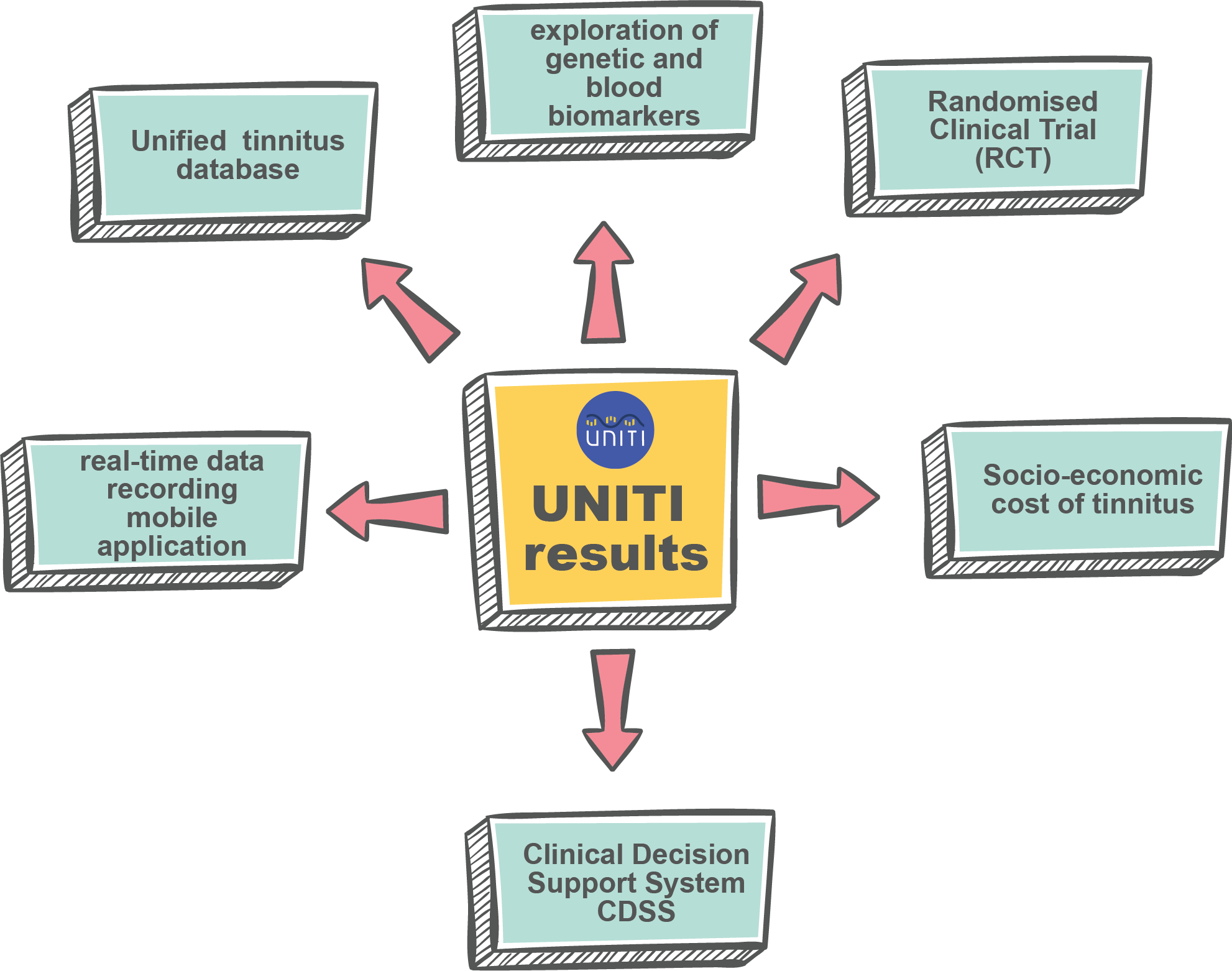
 We are happy to announce that we have a new paper published in Frontiers in Neuroscience: "
We are happy to announce that we have a new paper published in Frontiers in Neuroscience: " UNITI features at the
UNITI features at the 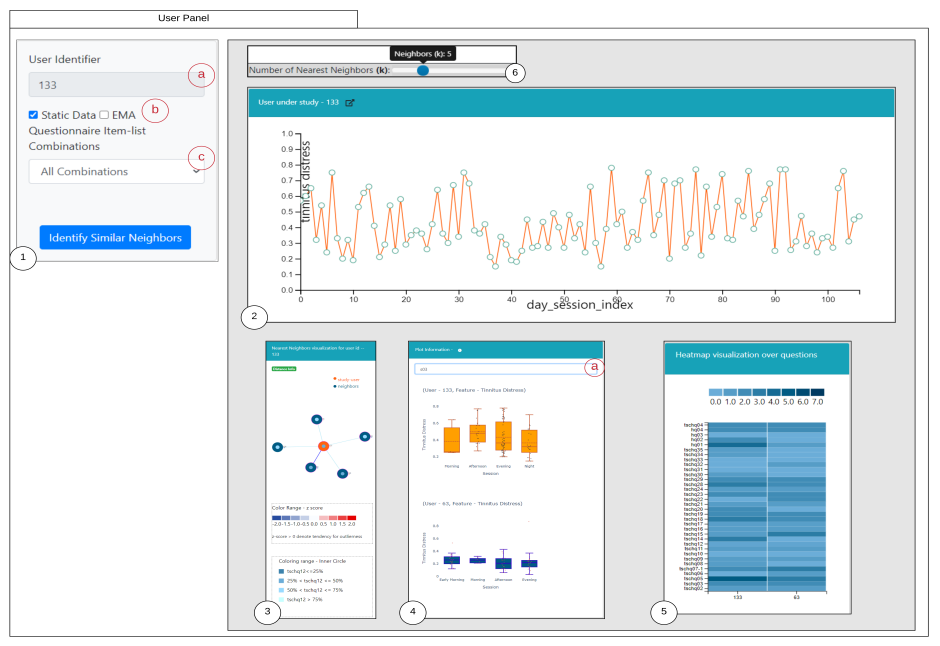 We are happy to announce that we have a new paper published in the MDPI Journal Entropy (Special Issue Challenges of Health Data Analytics). The publication is relevant from an mHealth prespective and its title is: "Interactive System for Similarity-Based Inspection and Assessment of the Well-Being of mHealth Users".
We are happy to announce that we have a new paper published in the MDPI Journal Entropy (Special Issue Challenges of Health Data Analytics). The publication is relevant from an mHealth prespective and its title is: "Interactive System for Similarity-Based Inspection and Assessment of the Well-Being of mHealth Users". We are happy to announce that we have a new paper published: "Discovery of Patient Phenotypes through Multi-layer Network Analysis on the Example of Tinnitus" and is available here:
We are happy to announce that we have a new paper published: "Discovery of Patient Phenotypes through Multi-layer Network Analysis on the Example of Tinnitus" and is available here: